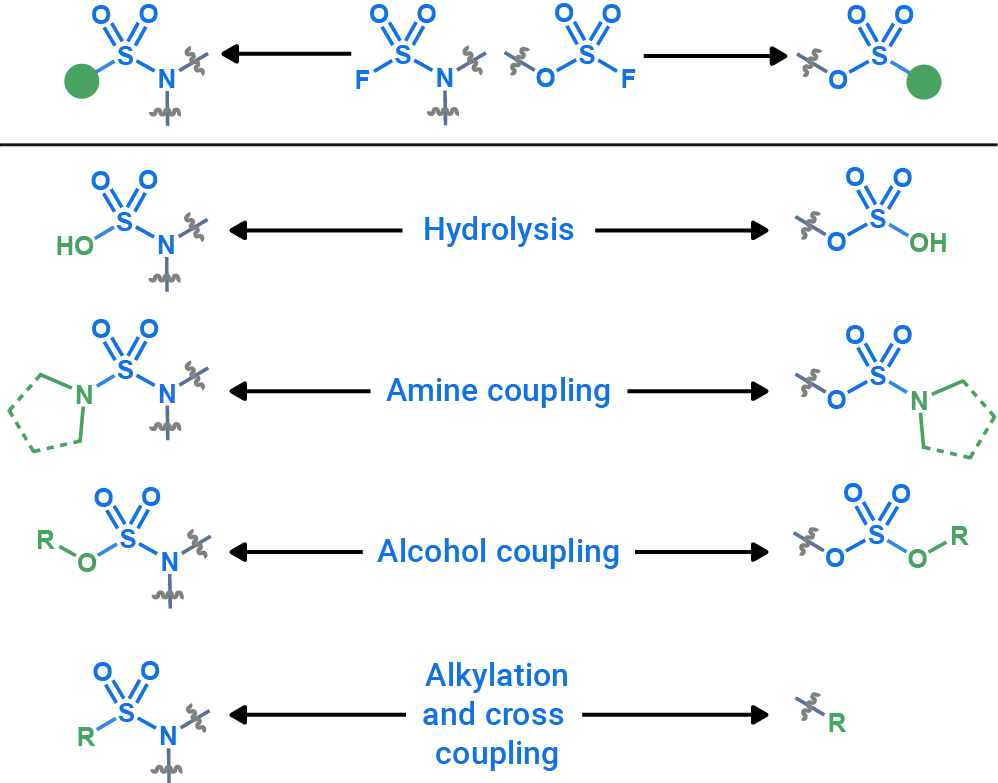
SuFEx handle fragment library
Sulfur-Fluorine Exchange: the second revolution of Click Chemistry
SuFEx is a family of click connective reactions based on the unique properties of the S(VI)–F bond to forge new linkages with nucleophiles under mild conditions.
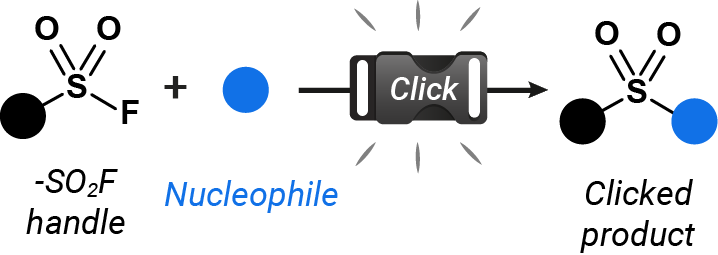
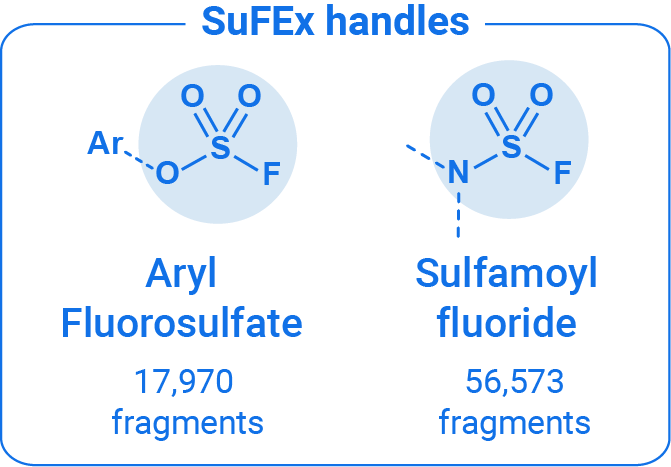
Through a collaboration between OTAVAchemicals and Melius Organics, we introduce a new SuFEx handle fragment library featuring 74,543 feasible fluorosulfate and sulfamoyl fluoride fragments.
Not only is this collection available for virtual screening, but these fragments can also be delivered physically, ensuring a seamless transition from computational exploration to tangible experimentation.
With all starting materials in stock, we guarantee fast preparation and delivery (typically within just three weeks) empowering your discovery pipeline with speed and reliability.
Applications in Medicinal Chemistry and Chemical Biology
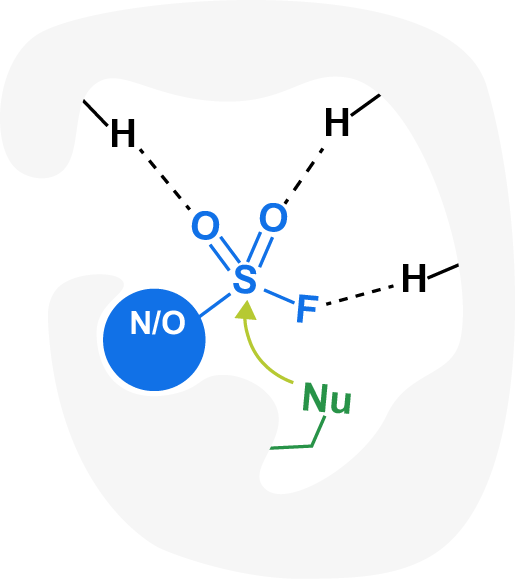
Aryl fluorosulfates and sulfamoyl fluorides are pivotal in medicinal chemistry as they can react with amioacids such as lysine, tyrosine, and histidine to form new covalent bonds. This makes these fragments suitable for covalent warheads inhibitors, enzyme profiling, protein crosslinking and peptide conjugation.
- “Masked electrophiles” activated in H-bonding environments
- High modification yields
- Good stability in blood plasma, microsomes, and hepetocites
- Fluoride leaving group safe up to mM
- Water-stable
- Not hampering cell permeability
Covalent inhibitors
Covalent drug discovery is at the vanguard of current medicinal chemistry and chemical biology.
While traditionally focused on targeting cysteine, the absence of this amino acid in many protein binding sites has led to the emergence of sulfur (VI) fluoride exchange (SuFEx) chemistry as a reliable alternative for expanding the druggable proteome.
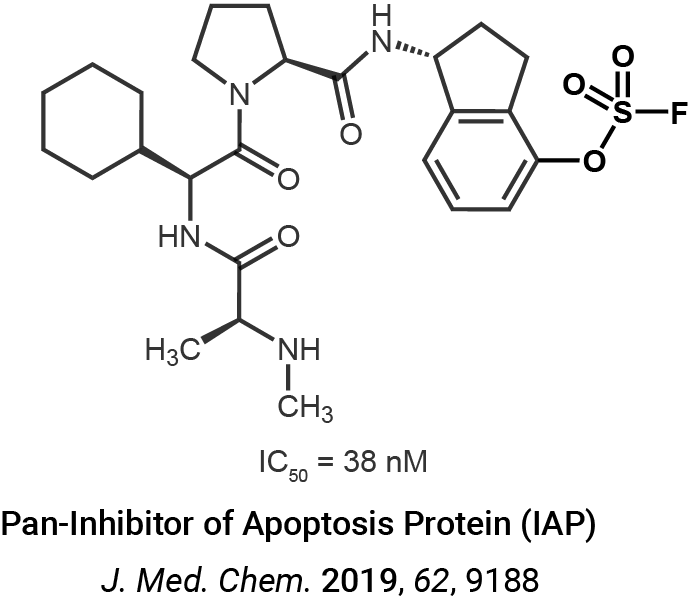
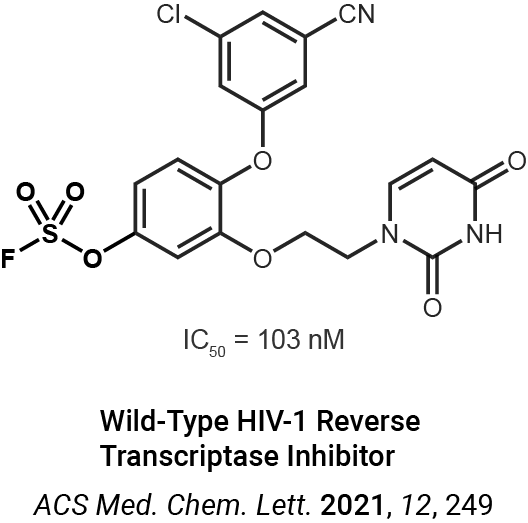
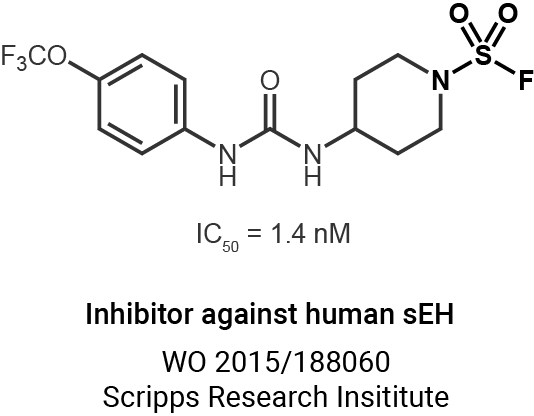
Enzyme profiling and proteomics
Covalent drug discovery is at the vanguard of current medicinal chemistry and chemical biology.
While traditionally focused on targeting cysteine, the absence of this amino acid in many protein binding sites has led to the emergence of sulfur (VI) fluoride exchange (SuFEx) chemistry as a reliable alternative for expanding the druggable proteome.
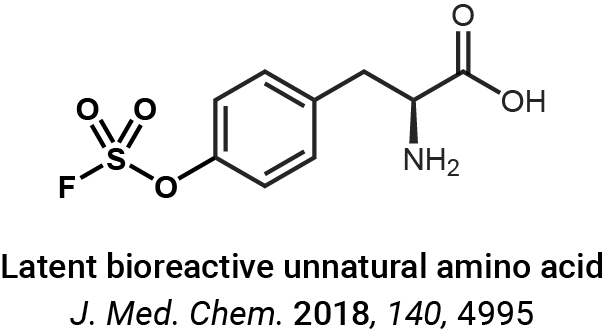
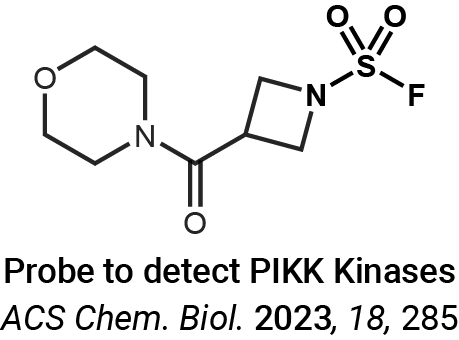
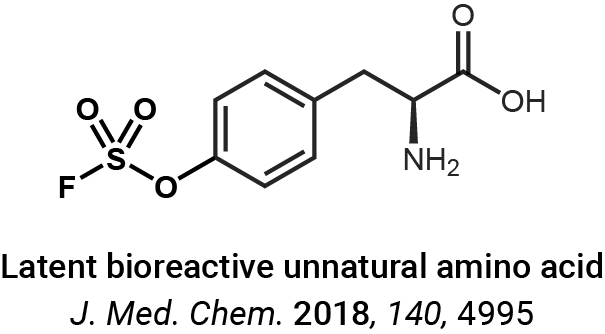
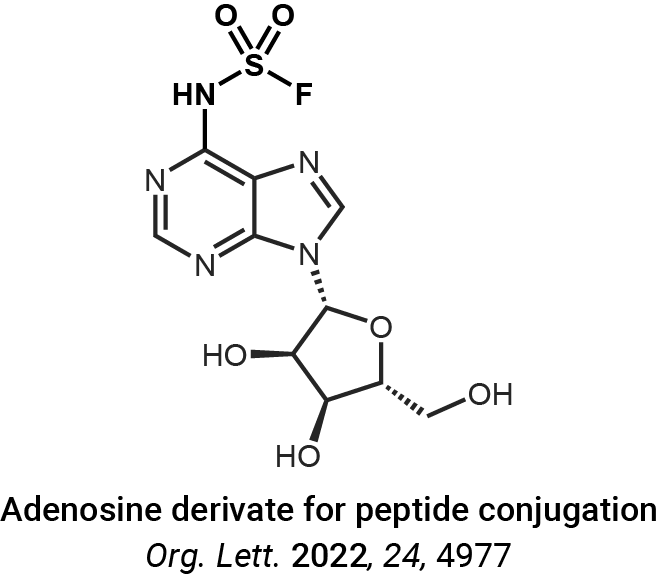
Protein crosslinking and peptide conjugation
Applications in Synthetic Chemistry
Fluorosulfates and sulfamoyl fluorides are versatile functional groups with significant applications in organic synthesis. Their “click chemistry” nature ensures high-yield reactions with minimal purification, making them reliable tools in building complex scaffolds.
These electrophilic functionalities can be activated under specific reaction conditions. Compared to their chlorinated analogs, fluorosulfates and sulfamoyl fluorides offer superior resistance to reduction, exceptional thermodynamic stability, and exclusive reactivity at the sulfur atom. Furthermore, they enable robust, high-yield reactions that are even compatible with aqueous media, making them particularly well-suited for late-stage functionalization due to their broad functional group tolerance.
Additionally, their ability to react with a diverse range of nucleophiles (O-, N-, and C-based) provides access to an expansive and varied chemical space.